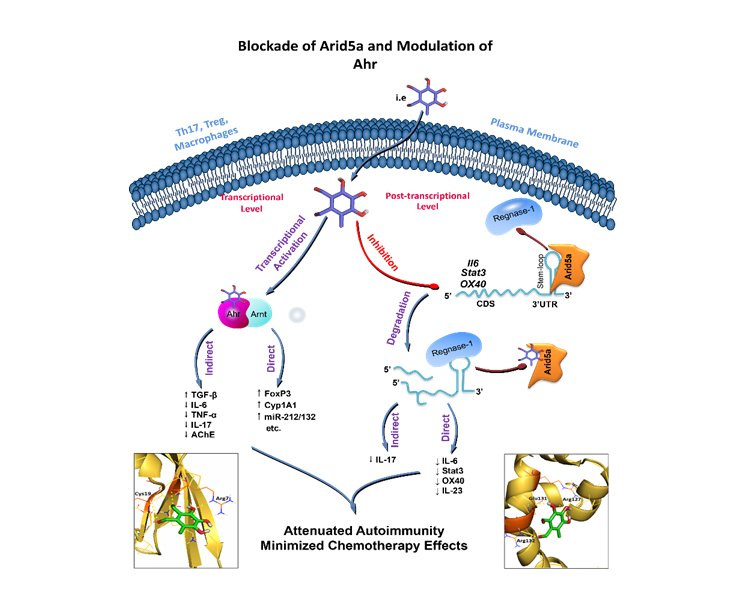
Our research programs are looking at both transcriptional and post-transcriptional regulatory levels during immune responses. We are perusing therapies in the following three targets:
Post-transcriptional Level
- Target 1: Blocking Arid5a, RNA-binding proteins (RBP), primarily for the treatment of multiple sclerosis and colon cancer.
- Target 2: Inhibiting RBP, identified by our hands, which binds mRNAs encoding cancer progression mediators and endows cancer stability.
Transcriptional Level
- Target 3: Modulation of Ahr transcriptional activities on downstream genes to minimize the side effects of chemotherapies.
Target 1: Blocking Arid5a for the treatment of multiple sclerosis and colon cancer.
Recently, the RNA-binding proteins (RBPs) have emerged as new players in the – development and progression of inflammatory and autoimmune disorders – owing to their ability to stabilize or destabilize target mRNAs through binding to their 3’UTR. Arid5a, an RNA-binding protein that has a unique function in the stabilization of mRNA. It is a dynamic molecule; upon inflammation, it translocate to the cytoplasm and stabilizes a variety of pro-inflammatory mediators encoding mRNAs. Our previous preclinical studies, outside iMReC, demonstrated that Arid5a stabilizes mRNAs encoding IL-6, IL-23a, STAT3, OX40 and T-bet. Thus contributing to the pathogenesis of several diseases such as multiple sclerosis
We were the first to identify the druggable pocket of Arid5a protein. We have designed med-sized peptides and several small molecules to inhibit Arid5a functions through this pocket (US patent, 10,709,639, 10,709,696, 11,001,608 and pending applications). We have shown that such Arid5a inhibitors exerted ameliorative effects in experimental models of multiple sclerosis, rheumatoid arthritis and psoriasis.
Recent studies emphasized the role of Arid5a in cancer progression/metastasis, including breast, pancreatic, colorectal, lung cancers and glioma. Although understanding the role of Arid5a in malignancies is still at its early stages, it is apt to suggest blockade of Arid5a as a new therapeutic strategy.
Blocking Arid5a in immune cells (macrophages and T cells) as a new therapeutic strategy is plausible. Through in-vitro assays, the team at iMReC is continuing to optimize several peptides and small molecules for their inhibitory capacities of Arid5a to create potent novel therapies for multiple sclerosis and colon cancers.
Target 2: Inhibiting RBP that endows stability to mRNAs encoding cancer progression mediator
The transcription factor SOX4 high expression is associated with poor prognosis in many cancers, in particular triple negative breast cancer. SOX4 promotes tumorigenesis by endowing cancer cells with survival, migratory, invasive and epithelial-to-mesenchymal transition (EMT) capacities. A paradigm of preclinical studies has suggested that inhibition of SOX4 is a promising strategy for control and treatment of tumors.
Similarly, the PD1, PD-L1 are immune checkpoints playing critical roles cancer progression and evasion. Clinical studies have shown the benefits of blocking PD1/PDL1 in controlling cancers. However, the number pf patients respond to these blockers is limited.
Although PD1/PDL1 and SOX4 are well-demonstrated factors promoting cancer, limited information is available about RBPs endowing stability to their mRNAs, and no inhibitors for these RBPs have been developed.
Using in-vitro experimental means, we are identifying candidate RBPs that bind to the 3’UTR of SOX4, CD279 and CD274 mRNAs and increase their half-lives. Subsequently, we will apply similar methodology to that used when identifying the Ari5a packet to develop inhibitors for these RBPs. Success in blockade of these tumor-promoting proteins will be a great addition to cancer immunotherapies.
Target 3: Modulation of Ahr to minimize the side effects of chemotherapies.
Currently, most of the patients are receiving chemotherapy and/or radiotherapy at high doses to control cancer progression and metastasis. Chemotherapeutic agents, however, are associated with frequent and serious side effects, which reduce patients’ quality of life and promote infections and bone marrow suppression. Thus, iMReC team is working on developing safe adjuvant therapy based on Ahr that modulates the immune system to allow lower doses of chemotherapeutic agents to be administered without loss of efficacy, but with improved tolerability.
Ahr is a ligand-activated transcription factor implicated in several aspects of autoimmune inflammation and cancer. It is expressed almost in all immune system cells such as macrophages, dendritic cells and T cells. The Ahr signaling pathway has been emerged among the potential druggable targets to treat and control progression of several cancers.
Based on our demonstrated work and discoveries in this field, we are manipulating Ahr transcriptional activity to create a novel therapy. In this program, we aim at optimizing and/or identifying Ahr agonist (natural and/or synthesized) to be used in combination with the available chemotherapeutic agents in cancer patients. We focus on colorectal cancer as it is common cancer and where Ahr is expressed in the colonic tumor tissues. It is expected that such therapy can be used in combination with chemotherapeutic agents not only for colorectal cancer patient, but also for many other cancer types.